
Chemistry, 19.11.2019 06:31 riveranikki2
The concentration of an aqueous solution of iron(ii) chloride is 0.0550 m. what is the molarity of each ion in this solution?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
The concentration of an aqueous solution of iron(ii) chloride is 0.0550 m. what is the molarity of e...
Questions

History, 25.07.2019 00:00

English, 25.07.2019 00:00

Mathematics, 25.07.2019 00:00

Mathematics, 25.07.2019 00:00


Social Studies, 25.07.2019 00:00

Mathematics, 25.07.2019 00:00

Mathematics, 25.07.2019 00:00



English, 25.07.2019 00:00

Mathematics, 25.07.2019 00:00



Biology, 25.07.2019 00:00

English, 25.07.2019 00:00


Biology, 25.07.2019 00:00


Mathematics, 25.07.2019 00:00

![[Fe^{2+} ]](/tpl/images/0380/9326/1243f.png) =0.0550 M
=0.0550 M![[Cl^{-} ]](/tpl/images/0380/9326/424e2.png) =0.110 M
=0.110 M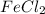 , thus upon ionizationm there will be two chloride anions for each iron cation:
, thus upon ionizationm there will be two chloride anions for each iron cation:![FeCl_{2} \rightarrow[Fe^{2+} ]+2[Cl^{-} ]](/tpl/images/0380/9326/15503.png)


