
Chemistry, 23.09.2019 23:30 sierra6816
How many moles of oxygen are necessary to completely react with propane (c3h8) to produce 16.3 mol of water according to the following balanced chemical equation? c3h8 + 5 o2 -> 3 co2 + 4 h2o
i tried doing these they are due by 12: 00 i have 2 more like this

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
You know the right answer?
How many moles of oxygen are necessary to completely react with propane (c3h8) to produce 16.3 mol o...
Questions


Geography, 20.04.2021 02:00

Health, 20.04.2021 02:00

English, 20.04.2021 02:00

Health, 20.04.2021 02:00



Mathematics, 20.04.2021 02:00


Mathematics, 20.04.2021 02:00






Spanish, 20.04.2021 02:00

Mathematics, 20.04.2021 02:00



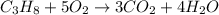
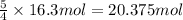 of oxygen
of oxygen

