
Calculate δh∘ in kilojoules for the reaction of ammonia nh3 (δh∘f=−46.1kj/mol) with o2 to yield nitric oxide (no) (δh∘f=91.3 kj/mol) and h2o(g) (δh∘f=−241.8kj/mol), a step in the ostwald process for the commercial production of nitric acid.
4nh3(g)+5o2(g)→4no(g)+6h2o(g)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
Calculate δh∘ in kilojoules for the reaction of ammonia nh3 (δh∘f=−46.1kj/mol) with o2 to yield nitr...
Questions




History, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01

English, 18.10.2020 06:01

English, 18.10.2020 06:01

History, 18.10.2020 06:01



Spanish, 18.10.2020 06:01


History, 18.10.2020 06:01


Mathematics, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01

Mathematics, 18.10.2020 06:01


Mathematics, 18.10.2020 06:01

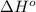 for the given reaction is -901.2 kJ.
for the given reaction is -901.2 kJ.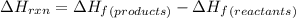
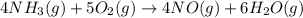
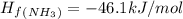
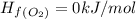
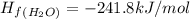
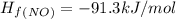
![\Delta H_{rxn}=[6\Delta H_f_{(H_2O)}+4\Delta H_f_{(H_2O)}-[4\Delta H_f_{(NH_3)}+5\Delta H_f_{(O_2)}]](/tpl/images/0307/6578/0d62c.png)
![\Delta H_{rxn}=[6mol(-241.8kJ/mol)+4mol(91.3kJ/mol)]-[4mol(-46.1kJ/mol)+5mol(0kJ/mol)]](/tpl/images/0307/6578/0673d.png)



