
Amolecule of carbon dioxide (co2) contains two polar covalent bonds. why is the molecule nonpolar?
each bond in the molecule is only moderately polar covalent.
the molecule is too small to be polar.
the polar bonds are oriented in opposite directions.
the carbon atom has two unshared pairs of electrons.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3


Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1
You know the right answer?
Amolecule of carbon dioxide (co2) contains two polar covalent bonds. why is the molecule nonpolar?...
Questions

Mathematics, 13.10.2020 06:01

Mathematics, 13.10.2020 06:01


Mathematics, 13.10.2020 06:01



Mathematics, 13.10.2020 06:01

English, 13.10.2020 06:01




Spanish, 13.10.2020 06:01


Mathematics, 13.10.2020 06:01



Biology, 13.10.2020 06:01


English, 13.10.2020 06:01

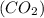 contains two polar covalent bonds. This molecule is non-polar because the polar bonds are oriented in the opposite direction.
contains two polar covalent bonds. This molecule is non-polar because the polar bonds are oriented in the opposite direction.

