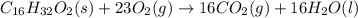Chemistry, 24.08.2019 10:10 princessjsl22
Palmitic acid c16h32o2 is a dietary fat found in beef and butter. the caloric content of palmitic acid is typical of fats in general.
part a
write a balanced equation for the complete combustion of palmitic acid. use \rm h_2o(l) in the balanced chemical equation because the metabolism of these compounds produces liquid water.
express your answer as a chemical equation. identify all of the phases in your answer.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1

You know the right answer?
Palmitic acid c16h32o2 is a dietary fat found in beef and butter. the caloric content of palmitic ac...
Questions


Mathematics, 11.07.2019 21:30



Chemistry, 11.07.2019 21:30






Mathematics, 11.07.2019 21:30

Mathematics, 11.07.2019 21:30

Geography, 11.07.2019 21:30

Mathematics, 11.07.2019 21:30


History, 11.07.2019 21:30

Business, 11.07.2019 21:30


English, 11.07.2019 21:30

Social Studies, 11.07.2019 21:30