
Chemistry, 10.11.2019 20:31 kiwipitts8661
If temperature increases from 65 degrees to 90 degrees, does the solubility increase or decrease for kbr during the same interval and by how much?
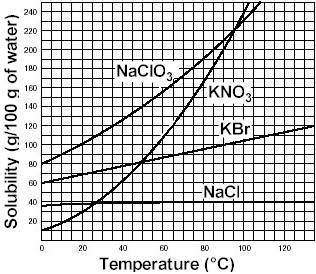

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 14:30
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1
You know the right answer?
If temperature increases from 65 degrees to 90 degrees, does the solubility increase or decrease for...
Questions

Arts, 24.01.2021 15:00






Social Studies, 24.01.2021 15:00

English, 24.01.2021 15:00

Arts, 24.01.2021 15:10


Mathematics, 24.01.2021 15:10

Chemistry, 24.01.2021 15:10

Physics, 24.01.2021 15:10

History, 24.01.2021 15:10

Health, 24.01.2021 15:10

History, 24.01.2021 15:10

Mathematics, 24.01.2021 15:10

Mathematics, 24.01.2021 15:10

Mathematics, 24.01.2021 15:10



