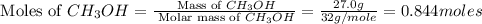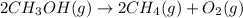Chemistry, 25.10.2019 18:43 samafeggins2
Consider the following reaction:
2ch3oh(g)→2ch4(g)+o2(g)δh=+252.8kj< br /> calculate the amount of heat transferred when 27.0g of ch3oh(g) is decomposed by this reaction at constant pressure.
if someone could me with the steps i can figure it out on my own, you so much

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 11:00
Which are examples of how technology has advanced scientific understanding.1using hot water to sterilize medical equipment.2transplanting a human organ into another individual.3inserting genes from one sheep into another cell to make a cloneunderstanding the different structures that make up a cell.4examining microorganisms from the deepest parts of the ocean
Answers: 2

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
Consider the following reaction:
2ch3oh(g)→2ch4(g)+o2(g)δh=+252.8kj< br /> calculate the amo...
2ch3oh(g)→2ch4(g)+o2(g)δh=+252.8kj< br /> calculate the amo...
Questions




English, 12.04.2021 23:40


English, 12.04.2021 23:40

Chemistry, 12.04.2021 23:50




English, 12.04.2021 23:50


Mathematics, 12.04.2021 23:50

Mathematics, 12.04.2021 23:50






History, 12.04.2021 23:50

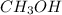 = 27.0 g
= 27.0 g