
Chemistry, 03.10.2019 12:50 andreastyles1603
Measurements show that enthalpy of a mixture of gaseous reactants decreases by 228. kj during a certain chemical reaction, which is carried out at a constant pressure. furthermore, by carefully monitoring the volume change it is determined that -55kj of work is done on the mixture during the reaction.
calculate the change in energy of the gas mixture during the reaction.
is the reaction exothermic or endothermic?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

Chemistry, 23.06.2019 14:00
Ahas distinct properties and composition that never vary.
Answers: 1
You know the right answer?
Measurements show that enthalpy of a mixture of gaseous reactants decreases by 228. kj during a cert...
Questions

Mathematics, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00

Computers and Technology, 17.11.2020 01:00

History, 17.11.2020 01:00

Chemistry, 17.11.2020 01:00


Biology, 17.11.2020 01:00

History, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00


Mathematics, 17.11.2020 01:00

History, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00

English, 17.11.2020 01:00



Mathematics, 17.11.2020 01:00

Mathematics, 17.11.2020 01:00

Geography, 17.11.2020 01:00

Biology, 17.11.2020 01:00

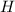 =-228 KJ
=-228 KJ 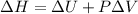
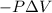
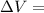 Change in volume
Change in volume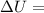 Change in energy
Change in energy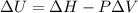

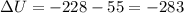 KJ
KJ

