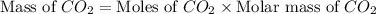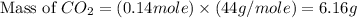You carefully weigh out 14.00 g of caco3 powder and add it to 56.70 g of hcl solution. you notice bubbles as a reaction takes place. you then weigh the resulting solution and find that it has a mass of 64.96 g . the relevant equation is
caco3(s)+2hcl(aq)→h2o(l)+co2(g)+cac l2(aq)
assuming no other reactions take place, what mass of co2 was produced in this reaction?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
You carefully weigh out 14.00 g of caco3 powder and add it to 56.70 g of hcl solution. you notice bu...
Questions

Geography, 23.06.2019 03:30

Advanced Placement (AP), 23.06.2019 03:30












Mathematics, 23.06.2019 03:30

History, 23.06.2019 03:30





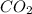 produced will be, 6.16 grams.
produced will be, 6.16 grams.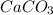 = 14 g
= 14 g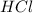 = 56.70 g
= 56.70 g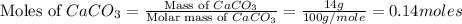
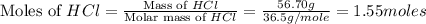
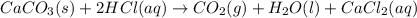
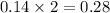 moles of
moles of