
Chemistry, 17.10.2019 04:30 Jacobolobo7
Consider this combination reaction:
2mg(s)+o2(g)→2mgo(s) δh=−1204 kj
what is the enthalpy for the decomposition of 1 mole of mgo(s) into mg(s) and o2(g)?
consider this combination reaction:
what is the enthalpy for the decomposition of 1 mole of into and ?
-1204 kj/mol
602 kj/mol
1204 kj/mol
-602 kj/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 21:30
Harry lives in a city, and he has a lung condition known as asthma. on certain days, harry has to stay inside because pollutants in the air make it difficult for him to breathe. which of these pollution sources are nonpoint sources that might bother harry if he goes outside? choose the two that apply.
Answers: 3
You know the right answer?
Consider this combination reaction:
2mg(s)+o2(g)→2mgo(s) δh=−1204 kj
what is the enthal...
2mg(s)+o2(g)→2mgo(s) δh=−1204 kj
what is the enthal...
Questions




Mathematics, 12.01.2021 07:30

Mathematics, 12.01.2021 07:30


Biology, 12.01.2021 07:30


Geography, 12.01.2021 07:30

Mathematics, 12.01.2021 07:30

English, 12.01.2021 07:30

Mathematics, 12.01.2021 07:30



Mathematics, 12.01.2021 07:30

Mathematics, 12.01.2021 07:30

Mathematics, 12.01.2021 07:30


English, 12.01.2021 07:30

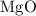 is
is 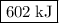 .
.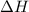 of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.
of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.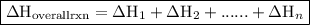
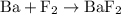
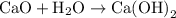
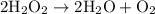
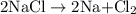
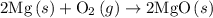
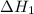 is
is 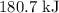 .
.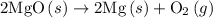
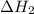 .
.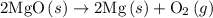 ......(2)
......(2)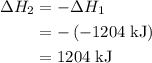
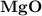 dissociates to give two moles of
dissociates to give two moles of 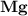 and one mole of
and one mole of 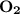 and therefore the enthalpy for the decomposition of one mole of is as follows:
and therefore the enthalpy for the decomposition of one mole of is as follows: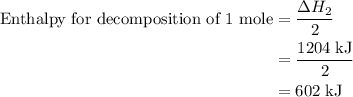
 .
.

