
Chemistry, 04.02.2020 20:48 arianaaldaz062002
Sort each of the following events based on whether the solubility of the indicated gas will increase, decrease, or stay the same. each phrase specifies the gas involved and the change in its environment.
put the following in one of three categories.
"gas solubility increases" gas solubility decreases" " gas solubility does not change"
1. the pressure of a gas over a solvent is increase
2. the partial pressure of an anesthetic gas is increased
3. air in blood a diver descends 10 m and pressure increases by 1 atm
4. the temp is increase
5. o2 the temp of a body of water rises.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 00:10
In as 1°, 2°, 3°, or 4°. be to . : °b: °c: °d: ° : °b: °c: °d: ° : °b: °c: °d: °e: °f: °g: °h: ° : °b: °c: °d: °e: °f: °g: °h: °i: °
Answers: 3
You know the right answer?
Sort each of the following events based on whether the solubility of the indicated gas will increase...
Questions



Mathematics, 07.03.2021 08:40


Mathematics, 07.03.2021 08:40


History, 07.03.2021 08:40

Mathematics, 07.03.2021 08:40

History, 07.03.2021 08:40

Biology, 07.03.2021 08:40


Biology, 07.03.2021 08:40

Mathematics, 07.03.2021 08:40


Mathematics, 07.03.2021 08:40

Mathematics, 07.03.2021 08:40

English, 07.03.2021 08:40

Physics, 07.03.2021 08:40

Social Studies, 07.03.2021 08:40

Mathematics, 07.03.2021 08:40

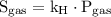
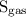 is the solubility of the gas.
is the solubility of the gas.
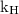 is Henry’s constant.
is Henry’s constant.
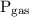 is the pressure of the gas.
is the pressure of the gas.


