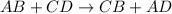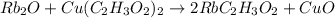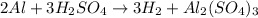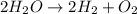Chemistry, 19.09.2019 01:00 seider7997
Which reaction below represents a balanced, double replacement chemical reaction? a) c2h5oh + 3o2 → 2co2 + 3h2o b) rb2o + cu(c2h3o2)2 → 2rbc2h3o2 + cuo c) 2al + 3h2so4 → 3h2 + al2(so4)3 eliminate d) 2h2o → 2h2 + o2

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

You know the right answer?
Which reaction below represents a balanced, double replacement chemical reaction? a) c2h5oh + 3o2 →...
Questions

Biology, 05.10.2019 18:50

Social Studies, 05.10.2019 18:50

Health, 05.10.2019 18:50



English, 05.10.2019 18:50


Computers and Technology, 05.10.2019 18:50


History, 05.10.2019 18:50

Mathematics, 05.10.2019 18:50

Health, 05.10.2019 18:50

English, 05.10.2019 18:50

Biology, 05.10.2019 18:50

Mathematics, 05.10.2019 18:50


Mathematics, 05.10.2019 18:50