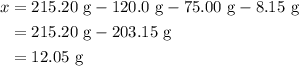Chemistry, 10.12.2019 23:31 sidallen05
When a solution of barium nitrate and a solution of copper (ii) sulfate are mixed, a chemical reaction produces solid barium sulfate, which sinks to the bottom, and a solution of copper (ii) nitrate. suppose some barium nitrate is dissolved in 120.00 g of water and 8.15 g of copper (ii) sulfate is dissolved in 75.00 g of water. the solutions are poured together, and a white solid forms. after the solid is filtered off, it is found to have a mass of 10.76 g. the mass of the solution that passed through the filter is 204.44 g. what mass of barium nitrate was used in the reaction?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Drag each tile to the correct box arrange the layers not order from oldest to youngest
Answers: 2

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 06:30
Select the correct text in the passage. which sentences describe examples of sustainable living? i live in an old apartment building downtown, but my company is based in an office park on the outskirts of the city. i drive an old car that needs to be replaced. i plan to buy a hybrid for better gas mileage, but for now i am able to carpool with a couple of friends from work. the drive to the office park is about 45 minutes each way, but we do get to work in a modern building. the architects just received a leed certification for the design.
Answers: 3

You know the right answer?
When a solution of barium nitrate and a solution of copper (ii) sulfate are mixed, a chemical reacti...
Questions





Mathematics, 13.02.2021 03:20

Biology, 13.02.2021 03:20



History, 13.02.2021 03:20

Mathematics, 13.02.2021 03:20


Mathematics, 13.02.2021 03:20

Health, 13.02.2021 03:20




Mathematics, 13.02.2021 03:20

Law, 13.02.2021 03:20


Mathematics, 13.02.2021 03:20

 .
.
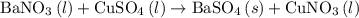
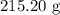 , and the mass of solid
, and the mass of solid 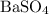 precipitate separated was
precipitate separated was 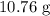 . Hence total mass of products is the sum of these two reactants.
. Hence total mass of products is the sum of these two reactants.
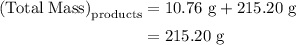
 . Mass of reactants involves mass of barium nitrate, water used for dissolving barium nitrate, mass of copper sulfate and water used for dissolving copper sulfate. The total mass of reactants is calculated as follows:
. Mass of reactants involves mass of barium nitrate, water used for dissolving barium nitrate, mass of copper sulfate and water used for dissolving copper sulfate. The total mass of reactants is calculated as follows:
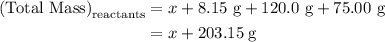
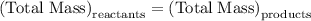 …… (1)
…… (1)
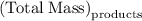 and
and 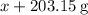 for
for 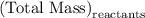 in equation
in equation 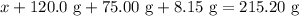
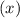 .
.