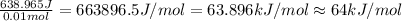Chemistry, 28.01.2020 03:31 uwunuzzles
Two solutions, initially at 24.69°c, are mixed in a coffee cup calorimeter (ccal = 105.5 j/°c). when a 200.0 ml volume of 0.100 m agno3 solution is mixed with a 100.0 ml sample of 0.100 m nacl solution, the temperature in the calorimeter rises to 25.16°c. determine the dh°rxn, in units of kj/mol agcl. assume that the density and heat capacity of the solutions is the same as that of water.
a. -78 kj/mol agcl
b. -25 kj/mol agcl
c. -64 kj/mol agcl
d. -32 kj/mol agcl
e. -59 kj/mol agcl

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 21:00
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2
You know the right answer?
Two solutions, initially at 24.69°c, are mixed in a coffee cup calorimeter (ccal = 105.5 j/°c). when...
Questions




Mathematics, 15.04.2020 02:57

Computers and Technology, 15.04.2020 02:57






English, 15.04.2020 02:57


Geography, 15.04.2020 02:58



History, 15.04.2020 02:58



Mathematics, 15.04.2020 02:58

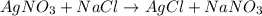
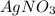 solution ,m'=
solution ,m'=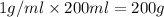
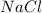 solution ,m''=
solution ,m''=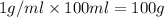
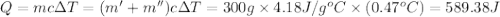
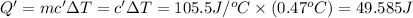
 =49.585 J+589.38 J=638.965 J
=49.585 J+589.38 J=638.965 J