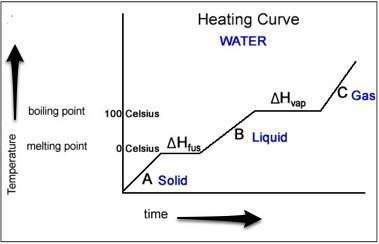
Describe the energy change in the particles of a substance during melting.
a) the kinetic ener...

Chemistry, 05.10.2019 00:30 niescarlosj
Describe the energy change in the particles of a substance during melting.
a) the kinetic energy of the particles remains unchanged.
b) absorbed energy results in the change in potential energy.
c) as the temperature increases during melting, the kinetic energy also increases.
d) the kinetic energy of the particles decreases as the particles move farther apart.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 22.06.2019 22:30
Consider a culture medium on which only gram-positive organisms such as staphylococcus aureus colonies can grow due to an elevated nacl level. a yellow halo surrounds the growth, indicating the bacterium fermented a sugar in the medium, decreasing the ph as a result and changing the color of a ph indicator chemical. this type of medium would be referred to as a differential and enrichment culture.
Answers: 2

Chemistry, 23.06.2019 03:30
Astudent uses universal ph paper to find the ph of three solutions . solution a has a ph of 5 solution b has a ph of 11 and solution c has a ph of 7 identify which solution is acidic which solution is neutral and which solution is basic
Answers: 1
You know the right answer?
Questions


History, 25.11.2019 18:31




Mathematics, 25.11.2019 18:31



Mathematics, 25.11.2019 18:31



English, 25.11.2019 18:31



Mathematics, 25.11.2019 18:31


History, 25.11.2019 18:31





