Which reaction will produce a solution that does not conduct electricity?
a. ch3oh(l) ch3oh(a...


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1



Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
Questions

Arts, 02.10.2020 14:01



Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01




Physics, 02.10.2020 14:01

Chemistry, 02.10.2020 14:01

Biology, 02.10.2020 14:01



Mathematics, 02.10.2020 14:01


Biology, 02.10.2020 14:01


Mathematics, 02.10.2020 14:01

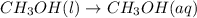 .
.

