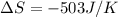Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 22.06.2019 20:30
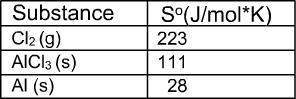
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
What is the value for ∆soreaction for the following reaction, given the standard entropy values? 2...
Questions








Chemistry, 14.02.2020 02:01

History, 14.02.2020 02:01











Computers and Technology, 14.02.2020 02:01

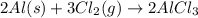
![\Delta S=\sum [n\times S^0(product)]-\sum [n\times \Delta S^0(reactant)]](/tpl/images/0286/6168/a3291.png)
![\Delta S=[(n_{AlCl_3}\times S_{AlCl_3})]-[(n_{Cl_2}\times S_{Cl_2})+[(n_{Al}\times S_{Al})]](/tpl/images/0286/6168/3ce6d.png)
![\Delta S=[(2\times 111)]-[(3\times 223)+(2\times 28)]](/tpl/images/0286/6168/a86a0.png)