
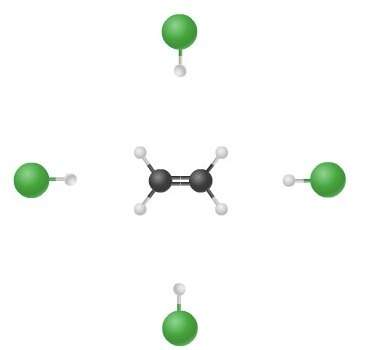
The illustration depicts possible routes of collisions in the reaction ch₂ch₂ + hcl ch₃ch₂cl. which of the following statements is true?
a. the chlorine atom does not participate in the reaction.
b. the hydrogen atom does not participate in the reaction.
c. the speed of the collision is essential.
d. the orientation of the reactants is critical.

Answers: 2
Another question on Chemistry

Chemistry, 20.06.2019 18:04
If this equation was completed which statement would it best support
Answers: 2

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2
You know the right answer?
The illustration depicts possible routes of collisions in the reaction ch₂ch₂ + hcl ch₃ch₂cl. which...
Questions


















Mathematics, 12.03.2020 23:06

Mathematics, 12.03.2020 23:07



