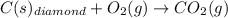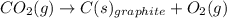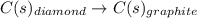Chemistry, 04.02.2020 06:51 hallynwright9722
When two intermediate chemical equations are combined, the same substance that appears in the same phase can be canceled out, provided that
a) it is a reactant in one intermediate reaction and a catalyst in the other reaction.
b) it is a product in one intermediate reaction and a catalyst in the other reaction.
c) it is a reactant in one intermediate reaction and a product in the other reaction.
d) it is a reactant in both of the intermediate reactions.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 18:30
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
When two intermediate chemical equations are combined, the same substance that appears in the same p...
Questions

Mathematics, 12.10.2019 08:10



Mathematics, 12.10.2019 08:10


History, 12.10.2019 08:10

Mathematics, 12.10.2019 08:10

Health, 12.10.2019 08:10


Chemistry, 12.10.2019 08:10

Mathematics, 12.10.2019 08:10





Mathematics, 12.10.2019 08:10

History, 12.10.2019 08:10

Physics, 12.10.2019 08:10

Business, 12.10.2019 08:10

Chemistry, 12.10.2019 08:10