
D: when sodium sulfide dissociates in water, the sulfur ion reacts with water as follows: s-2 + h2o → oh- + sh- which of the following statements is true? na2s is a base because it ionizes to release oh-. na2s is an acid because it is a proton donor. na2s is a base because it increases the hydroxide concentration. none of these

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
D: when sodium sulfide dissociates in water, the sulfur ion reacts with water as follows: s-2 + h2...
Questions




History, 06.04.2021 01:00





Mathematics, 06.04.2021 01:00

Mathematics, 06.04.2021 01:00

History, 06.04.2021 01:00


Biology, 06.04.2021 01:00






Mathematics, 06.04.2021 01:00

Social Studies, 06.04.2021 01:00

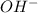 ) upon dissociation in water. This means that a base increases the hydroxide ion concentration in a solution.
) upon dissociation in water. This means that a base increases the hydroxide ion concentration in a solution. 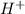 ) upon dissociation in water. This means that an acid increases the hydrogen ion concentration in a solution.
) upon dissociation in water. This means that an acid increases the hydrogen ion concentration in a solution.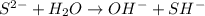
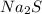 ) dissociates to give hydroxide ions.
) dissociates to give hydroxide ions. 

