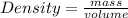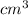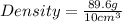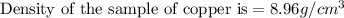Chemistry, 13.01.2020 23:31 charnaetoney13
Daniel has a sample of pure copper. its mass is 89.6 grams (g), and its volume is 10 cubic centimeters (cm3). what’s the density of the sample?
0.11 g/cm3
8.96 g/cm3
11.1 g/cm3
896 g/cm3

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 23.06.2019 01:30
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1
You know the right answer?
Daniel has a sample of pure copper. its mass is 89.6 grams (g), and its volume is 10 cubic centimete...
Questions

Mathematics, 19.08.2019 06:50

Mathematics, 19.08.2019 06:50



Mathematics, 19.08.2019 06:50

Chemistry, 19.08.2019 06:50


Mathematics, 19.08.2019 06:50

Biology, 19.08.2019 06:50


Social Studies, 19.08.2019 06:50

Social Studies, 19.08.2019 06:50

Social Studies, 19.08.2019 06:50



Mathematics, 19.08.2019 06:50



Mathematics, 19.08.2019 06:50