
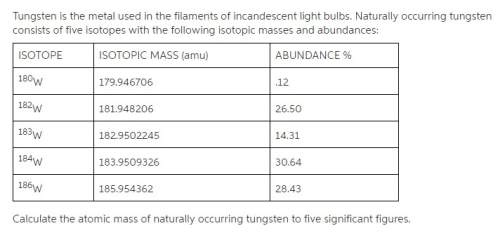
Tungsten is the metal used in the filaments of incandescent light bulbs. naturally occurring tungsten consists of five isotopes with the following isotopic masses and abundances:
calculate the atomic mass of naturally occurring tungsten to five significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1

Chemistry, 23.06.2019 07:30
The compound formed from 2 atoms of hydrogen and one atom of oxygen is
Answers: 1
You know the right answer?
Tungsten is the metal used in the filaments of incandescent light bulbs. naturally occurring tungste...
Questions

Mathematics, 05.10.2020 03:01




Mathematics, 05.10.2020 03:01




Mathematics, 05.10.2020 03:01

Mathematics, 05.10.2020 03:01

English, 05.10.2020 03:01


Mathematics, 05.10.2020 03:01

History, 05.10.2020 03:01



Geography, 05.10.2020 03:01


Mathematics, 05.10.2020 03:01



