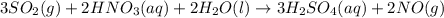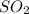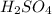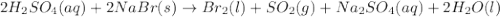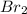Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3


Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1
You know the right answer?
Identify the elements that undergo changes in oxidation number in the reactions:
•nai(a...
•nai(a...
Questions

History, 20.09.2019 15:30



Physics, 20.09.2019 15:30


History, 20.09.2019 15:30



Mathematics, 20.09.2019 15:30



Biology, 20.09.2019 15:30

Social Studies, 20.09.2019 15:30


Physics, 20.09.2019 15:30

English, 20.09.2019 15:30

History, 20.09.2019 15:30



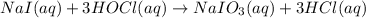
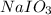 , the oxidation state of iodine is +5.
, the oxidation state of iodine is +5.