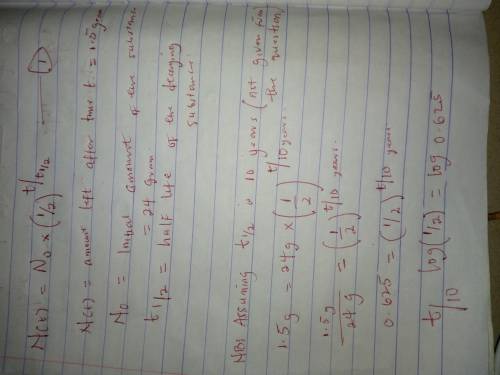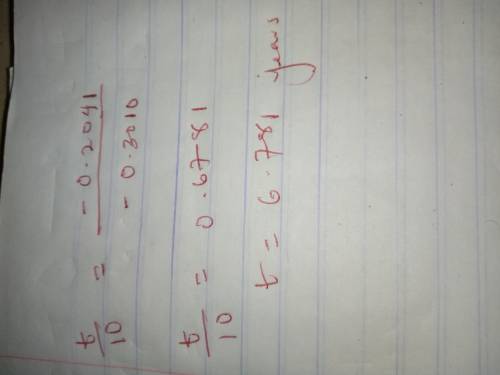Chemistry, 04.02.2020 17:03 qwertylol12345
Determine the time required for an original 24.0-gram sample of sr-94 to decay until only 1.5 grams of the sample remains unchanged.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Select the correct answer. you have a nightlight plugged into an outlet in the hallway, which uses 3.5 watts when plugged in. if the house circuit provides 120.0 volts, what is the current through this bulb?
Answers: 1

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
Determine the time required for an original 24.0-gram sample of sr-94 to decay until only 1.5 grams...
Questions

Mathematics, 29.04.2021 06:10






Biology, 29.04.2021 06:10

French, 29.04.2021 06:10


Law, 29.04.2021 06:10



Mathematics, 29.04.2021 06:10

Mathematics, 29.04.2021 06:10

Mathematics, 29.04.2021 06:10


Mathematics, 29.04.2021 06:10


Social Studies, 29.04.2021 06:10

Computers and Technology, 29.04.2021 06:10