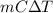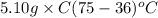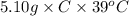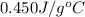Chemistry, 21.12.2019 17:31 nakarelinp0p303
A5.10 g sample of iron is heated from 36.0 to 75.0 c. the amount of energy required is 89.5 j. the specific capacity of this sample of iron
a.) 17800 j/g c
b.)0.900 j/g c
c.)11.7 j/g c
d.) 0.450 j/g c
e.) 2.22 j/g c

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
You know the right answer?
A5.10 g sample of iron is heated from 36.0 to 75.0 c. the amount of energy required is 89.5 j. the s...
Questions






Social Studies, 09.04.2020 04:32

Mathematics, 09.04.2020 04:32






History, 09.04.2020 04:32



Mathematics, 09.04.2020 04:32

English, 09.04.2020 04:32


History, 09.04.2020 04:32

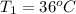 ,
,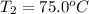 , q = 89.5 J
, q = 89.5 J