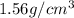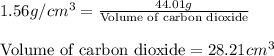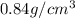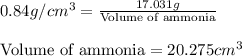Chemistry, 18.09.2019 06:50 naidaisha18
The densities of crystalline co2 and nh3 at 160 k are 1.56 and 0.84 g/cm3, respectively. calculate their molar volumes.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1

Chemistry, 23.06.2019 09:20
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1

Chemistry, 23.06.2019 12:30
An atom holds 7 electrons. use orbital notation to model the probable location of its electrons. an atom hold 22 electrons. use orbital notation to model the probable location of its electrons. an atom holds 17 electrons. use orbital notation to model the probable location of its electrons.
Answers: 1
You know the right answer?
The densities of crystalline co2 and nh3 at 160 k are 1.56 and 0.84 g/cm3, respectively. calculate t...
Questions

Mathematics, 17.09.2019 22:40

Mathematics, 17.09.2019 22:40

Mathematics, 17.09.2019 22:40

Physics, 17.09.2019 22:40

Advanced Placement (AP), 17.09.2019 22:40


Social Studies, 17.09.2019 22:40




History, 17.09.2019 22:40



History, 17.09.2019 22:40


Mathematics, 17.09.2019 22:40


Biology, 17.09.2019 22:40


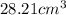 and that of ammonia is
and that of ammonia is 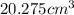
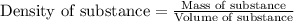 ......(1)
......(1)