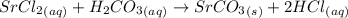Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
Write a balanced net ionic equation for the following reaction: srcl2(aq) + h2co3(aq) → srco3(s) +...
Questions

Biology, 15.12.2019 02:31



Spanish, 15.12.2019 02:31


Mathematics, 15.12.2019 02:31

Mathematics, 15.12.2019 02:31

Chemistry, 15.12.2019 02:31


Mathematics, 15.12.2019 02:31


Mathematics, 15.12.2019 02:31

Mathematics, 15.12.2019 02:31

Mathematics, 15.12.2019 02:31


Mathematics, 15.12.2019 02:31




Mathematics, 15.12.2019 02:31