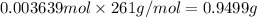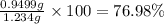The concentration of barium ions in any solution can also be determined via gravimetric analysis. an impure sample of barium nitrate with a mass of 1.234 g, is completely dissolved in water and the resulting solution is reacted with an excess of aqueous sodium sulfate. a precipitate forms, and after filtering and drying, it was found to have a mass of 0.848 g.
a) what is the relevance of adding eccess sodium sulfate?
b) calculate the % of barium nitrate in the original 1.234 g sample.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1


Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
You know the right answer?
The concentration of barium ions in any solution can also be determined via gravimetric analysis. an...
Questions


English, 27.02.2021 22:30

Mathematics, 27.02.2021 22:30

Business, 27.02.2021 22:30


Mathematics, 27.02.2021 22:30

Biology, 27.02.2021 22:30

English, 27.02.2021 22:30


Physics, 27.02.2021 22:30


Business, 27.02.2021 22:30


Mathematics, 27.02.2021 22:30


Chemistry, 27.02.2021 22:30

Mathematics, 27.02.2021 22:30



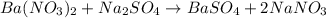
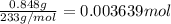
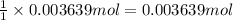 of barium nitrate.
of barium nitrate.