The heating curve below represents a sample of
a substance starting as a solid below its melti...

The heating curve below represents a sample of
a substance starting as a solid below its melting
point and being heated over a period of time.
which statement describes the energy of the
particles in this sample during interval de?
(1) both potential energy and average kinetic
energy increase.
(2) both potential energy and average kinetic
energy decrease.
(3) potential energy increases and average
kinetic energy remains the same.
(4) potential energy remains the same and
average kinetic energy increases.
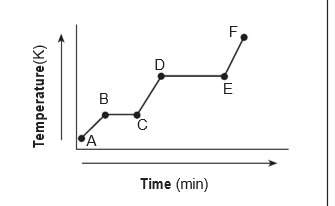

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3
You know the right answer?
Questions

Mathematics, 30.01.2020 22:43

Mathematics, 30.01.2020 22:43




History, 30.01.2020 22:43

English, 30.01.2020 22:43





Social Studies, 30.01.2020 22:43




Mathematics, 30.01.2020 22:43

History, 30.01.2020 22:43

Biology, 30.01.2020 22:43

Mathematics, 30.01.2020 22:43



