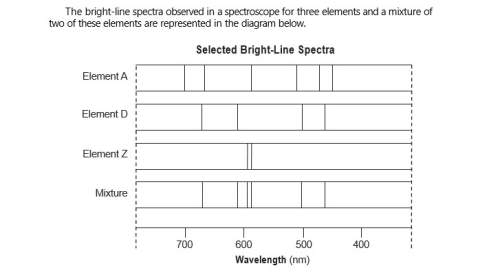
Describe, in terms of both electrons and energy states, how the light represented by
the spect...

Chemistry, 26.12.2019 06:31 perezshayla56
Describe, in terms of both electrons and energy states, how the light represented by
the spectral lines is produced.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
Questions




Computers and Technology, 03.02.2021 01:00

History, 03.02.2021 01:00





Arts, 03.02.2021 01:00


Mathematics, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00



Mathematics, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00




