Identify the noble gas that has atoms with the same electron configuration as the
positive ion...

Chemistry, 07.01.2020 11:31 brandistrothma
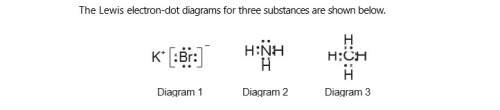
Identify the noble gas that has atoms with the same electron configuration as the
positive ion represented in diagram 1, when both the atoms and the ion are in the
ground state.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1
You know the right answer?
Questions














Computers and Technology, 01.04.2020 16:47





Mathematics, 01.04.2020 16:48



