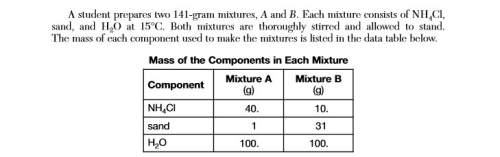
Determine the temperature at which all of the nh4cl in mixture a dissolves to form a
saturated...


Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 21.06.2019 22:30
Which statement best describes the oxidation numbers of the atoms found in magnesium chloride? a. magnesium has a 2- oxidation number and chlorine has a 1+ oxidation number. b. magnesium has a 2- oxidation number and chlorine has a 2+ oxidation number. c. magnesium has a 2+ oxidation number and chlorine has a 1- oxidation number. d. magnesium has a 1+ oxidation number and chlorine has a 1- oxidation number.
Answers: 2

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3
You know the right answer?
Questions



English, 19.11.2020 01:00

Mathematics, 19.11.2020 01:00

Law, 19.11.2020 01:00

SAT, 19.11.2020 01:00


Mathematics, 19.11.2020 01:00

Chemistry, 19.11.2020 01:00

Biology, 19.11.2020 01:00

English, 19.11.2020 01:00

Computers and Technology, 19.11.2020 01:00



Mathematics, 19.11.2020 01:00

History, 19.11.2020 01:00

English, 19.11.2020 01:00

History, 19.11.2020 01:00