
Chemistry, 05.10.2019 06:30 juniorcehand04
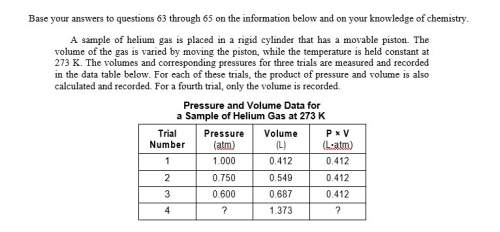
Asample of helium gas is placed in a rigid cylinder that has a movable piston. the volume of the gas is varied by moving the piston, while the temperature is held constant at 273 k. the volumes and corresponding pressures for three trials are measured and recorded in the data table below. for each of these trials, the product of pressure and volume is also calculated and recorded. for a fourth trial, only the volume is recorded.
state evidence found in the data table that allows the product of pressure and volume for the fourth trial to be predicted.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1
You know the right answer?
Asample of helium gas is placed in a rigid cylinder that has a movable piston. the volume of the gas...
Questions



Mathematics, 11.03.2020 00:02



Mathematics, 11.03.2020 00:02



Business, 11.03.2020 00:02

History, 11.03.2020 00:02

History, 11.03.2020 00:02

History, 11.03.2020 00:02

History, 11.03.2020 00:02





English, 11.03.2020 00:02

Physics, 11.03.2020 00:02

History, 11.03.2020 00:02



