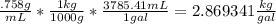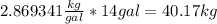Chemistry, 22.10.2019 16:00 GracieMaeB
E85 fuel is a mixture of 85% ethanol and 15% gasoline by volume. what mass in kilograms of e85(d=0.758g/ml) can be continued in 14.0- gal tank. show how to get answer.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1
You know the right answer?
E85 fuel is a mixture of 85% ethanol and 15% gasoline by volume. what mass in kilograms of e85(d=0.7...
Questions

Social Studies, 03.11.2020 18:50

Mathematics, 03.11.2020 18:50





History, 03.11.2020 18:50

Mathematics, 03.11.2020 18:50



Chemistry, 03.11.2020 18:50


Mathematics, 03.11.2020 18:50


History, 03.11.2020 18:50

Geography, 03.11.2020 18:50


Arts, 03.11.2020 18:50

Mathematics, 03.11.2020 18:50

Computers and Technology, 03.11.2020 18:50