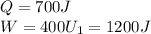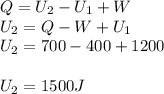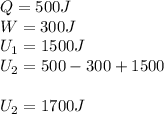Chemistry, 23.12.2019 07:31 Victoriag2626
In a heat engine, if 700 j of heat enters the system, and the piston does 400 j of work, what is the final internal (thermal) energy of the system if the initial energy is 1,200 j?
1,100 j
900 j
1,500 j
300 j
in a heat engine, if 500 j of heat enters the system, and the piston does 300 j of work, what is the final internal (thermal) energy of the system if the initial energy is 1,500 j?
1,700 j800 j1,300 j200 j

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:40
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
In a heat engine, if 700 j of heat enters the system, and the piston does 400 j of work, what is the...
Questions





Mathematics, 20.09.2021 05:40

Mathematics, 20.09.2021 05:40

Geography, 20.09.2021 05:40

Mathematics, 20.09.2021 05:40

Mathematics, 20.09.2021 05:40


History, 20.09.2021 05:40


Chemistry, 20.09.2021 05:40


English, 20.09.2021 05:40



History, 20.09.2021 05:40