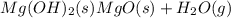Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aspirin has a density of 1.40 g/cm3 what is the volume in cubic centimeters of a tablet weighing 320 mg ?
Answers: 1

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

You know the right answer?
Solid magnesium hydroxide decomposes into solid magnesium oxide and gaseous water . write a balanced...
Questions


Spanish, 14.12.2020 21:10

Mathematics, 14.12.2020 21:10

Mathematics, 14.12.2020 21:10


Mathematics, 14.12.2020 21:10

English, 14.12.2020 21:10

History, 14.12.2020 21:10

Mathematics, 14.12.2020 21:10

Computers and Technology, 14.12.2020 21:10

Mathematics, 14.12.2020 21:10








Mathematics, 14.12.2020 21:10