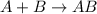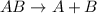Given the balanced equation representing a reaction:
zn(s) + h2so4(aq) → znso4(aq) + h2...

Chemistry, 03.11.2019 18:31 naomicervero
Given the balanced equation representing a reaction:
zn(s) + h2so4(aq) → znso4(aq) + h2(g)
which type of reaction is represented by this equation?
1.
decomposition
2.
double replacement
3.
single replacement
4.
synthesis

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2
You know the right answer?
Questions

Mathematics, 16.04.2020 00:15


Mathematics, 16.04.2020 00:15

Mathematics, 16.04.2020 00:15



Biology, 16.04.2020 00:15

English, 16.04.2020 00:15

Mathematics, 16.04.2020 00:15

Mathematics, 16.04.2020 00:15



Mathematics, 16.04.2020 00:15



History, 16.04.2020 00:15


History, 16.04.2020 00:15

Mathematics, 16.04.2020 00:15

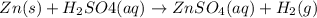 is a single replacement reaction.
is a single replacement reaction.