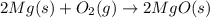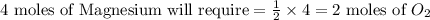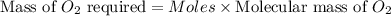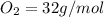Chemistry, 23.01.2020 14:31 blessed4628
The chemical equation below shows the burning of magnesium (mg) with oxygen (o2) to form magnesium oxide (mgo).
2mg + o2 mc009-1.jpg 2mgo
the molar mass of o2 is 32.0 g/mol. what mass, in grams, of o2 is required to react completely with 4.00 mol of mg?
2.00

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Zinc + lead(ii) nitrate yield zinc nitrate + leadwhat's the chemical equation for this?
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1


Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1
You know the right answer?
The chemical equation below shows the burning of magnesium (mg) with oxygen (o2) to form magnesium o...
Questions





Mathematics, 17.07.2019 11:00

Mathematics, 17.07.2019 11:00







Mathematics, 17.07.2019 11:00

English, 17.07.2019 11:00


Mathematics, 17.07.2019 11:00

Mathematics, 17.07.2019 11:00

Mathematics, 17.07.2019 11:00

Mathematics, 17.07.2019 11:00

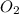 will be required to react completely with 4 moles of Mg.
will be required to react completely with 4 moles of Mg.