
Chemistry, 31.01.2020 23:01 jaymoney0531
Which statement best describes a bronsted-lowry base?
a) it must accept protons to form a conjugate base.
b) it must accept protons to form a conjugate acid.
c) it must donate protons to form a conjugate base.
d) it must donate protons to form a conjugate acid.

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2

Chemistry, 23.06.2019 04:00
The movement of tectonic plates and in two locations is described below: location a: tectonic played push together location b: tectonic plates push apart
Answers: 1

Chemistry, 23.06.2019 06:00
When hydrogen peroxide (h2o2) is added to potassium iodide (ki) solution, the hydrogen peroxide decomposes into water (h2o) and oxygen (o2). the chemical equation for the decomposition reaction is: 2h2o2—> 2h2o + o2. what is the role of the potassium iodide in this reaction? a. reactant. b. product. c. precipitate. d. catalyst.
Answers: 1

Chemistry, 23.06.2019 07:30
How do you interpret a chromagram for what mixtures contain?
Answers: 1
You know the right answer?
Which statement best describes a bronsted-lowry base?
a) it must accept protons to form a con...
a) it must accept protons to form a con...
Questions

History, 14.01.2020 18:31



Mathematics, 14.01.2020 18:31



Mathematics, 14.01.2020 18:31

Mathematics, 14.01.2020 18:31

History, 14.01.2020 18:31




Mathematics, 14.01.2020 18:31





Mathematics, 14.01.2020 18:31


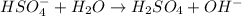
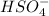 is a Bronsted-Lowry base because it can accept proton or hydrogen ion from
is a Bronsted-Lowry base because it can accept proton or hydrogen ion from 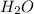 .
. 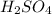 as conjugate acid.
as conjugate acid.


