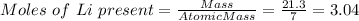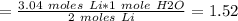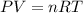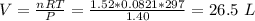Chemistry, 23.12.2019 22:31 terrysizemore666
If 21.3 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 297 kelvin and 1.40 atmospheres? show all of the work used to solve this problem. 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 13:30
Consider this reaction taking place in a closed 2 liter container: 2so2(g) + o2(g) → 2so3(g) if the volume of the container is decreased to 1 liter, what will happen to the equilibrium of the reaction? it will shift left. it will shift right. it will remain constant it will decrease by half
Answers: 3
You know the right answer?
If 21.3 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at...
Questions


English, 03.05.2021 06:50

Biology, 03.05.2021 06:50

Mathematics, 03.05.2021 06:50






Biology, 03.05.2021 06:50

Social Studies, 03.05.2021 06:50

Mathematics, 03.05.2021 06:50


Mathematics, 03.05.2021 06:50

Physics, 03.05.2021 06:50

Biology, 03.05.2021 06:50


Health, 03.05.2021 06:50


Chemistry, 03.05.2021 06:50