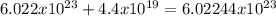Chemistry, 07.01.2020 16:31 milkshakegrande101
In chemistry, 6.022× 1023 is an important number called the avogadro constant (or avogadro\'s number). it expresses the number of atoms or molecules in a mole. notice that it is a really, really big number, as indicated by scientific notation with a large positive exponent. enter 6.022× 1023 into the calculator shown here. then, by trial and error, find the largest number that can be added to 6.022× 1023 without changing its displayed value (as shown on the screen).

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1


Chemistry, 23.06.2019 05:00
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2

Chemistry, 23.06.2019 10:30
Ethyl alcohol, also known as ethanol, has a density of 0.79 g/ml. what is the volume, in quarts, of 1.95 kg of this alcohol?
Answers: 2
You know the right answer?
In chemistry, 6.022× 1023 is an important number called the avogadro constant (or avogadro\'s number...
Questions

History, 19.07.2019 18:30


Mathematics, 19.07.2019 18:30

English, 19.07.2019 18:30



World Languages, 19.07.2019 18:30


Health, 19.07.2019 18:30


Biology, 19.07.2019 18:30



Biology, 19.07.2019 18:30