
Aphoton has a wavelength of 6.2 meters. calculate the energy of the photon in joules. (planck's constant is 6.626 × 10-34 joule seconds, the speed of light is 2.998 × 108 m/s) 3.564 × 10-43 joules 3.204 × 10-26 joules 41.08 × 10-34 joules 13.702 × 10-42 joules
answer b

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1


Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
You know the right answer?
Aphoton has a wavelength of 6.2 meters. calculate the energy of the photon in joules. (planck's cons...
Questions




Mathematics, 04.06.2021 07:30



Mathematics, 04.06.2021 07:30


Chemistry, 04.06.2021 07:30

Mathematics, 04.06.2021 07:30


Mathematics, 04.06.2021 07:30

Mathematics, 04.06.2021 07:30

Mathematics, 04.06.2021 07:30

Mathematics, 04.06.2021 07:30


Mathematics, 04.06.2021 07:30

Biology, 04.06.2021 07:30

English, 04.06.2021 07:30

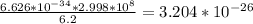 J. Hope this helps you!
J. Hope this helps you!

