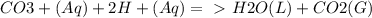Chemistry, 21.09.2019 08:50 yarrito20011307
Every antacid contains one or more ingredients capable of reacting with excess stomach acid (hcl). the essential neutralization products are co2 and/or h2o. write net ionic equations to represent the neutralizing action of the following popular antacids:
-rolaids
-maalox
-tums
-milk of magnesia

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 05:50
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

You know the right answer?
Every antacid contains one or more ingredients capable of reacting with excess stomach acid (hcl). t...
Questions

English, 18.02.2021 23:00



Mathematics, 18.02.2021 23:00






Mathematics, 18.02.2021 23:00

English, 18.02.2021 23:00


English, 18.02.2021 23:00

Mathematics, 18.02.2021 23:00


Computers and Technology, 18.02.2021 23:00