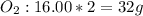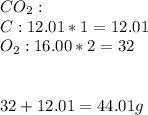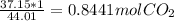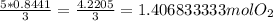1.407 mol O2
Explanation:
1) First, let's find the molar masses of O2 and CO2 by using the periodic table.
O2: 32g
Use the periodic table to find the average atomic mass of oxygen (O), which is 16.00. Since there are atoms of oxygen in O2, multiply 16(2) to get the molar mass of 32 g.
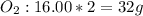
CO2: 44.01 g
Use the periodic table to find the average atomic mass of carbon (C), which is 12.01. There is one atom of carbon in CO2, and two atoms of oxygen. We solved earlier that the molar mass of O2 was 32g, so we can simply add 12.01+32 to get the molar mass of 44.01g.
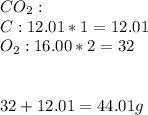
2) Set up a proportion (I will be using a shortcut method) to find the number of moles of CO2 in 37.15 g of CO2.
We know that 1 mol of CO2 has a molar mass of 44.01 g. We need to find the number of moles of CO2 in 37.15 g. This is how I like to set up the proportion:
1 mol CO2 - 44.01 g CO2
x mol CO2 - 37.15 g CO2
As you can see, the proportion shows that 1 mol of CO2 corresponds to 44.01g of CO2. Additionally, x moles of CO2 corresponds with 37.15g of CO2. x mol represents the variable that we need to find.
*Make sure that the units are the same on the left and right sides. In this example, moles are all on the left and grams are all on the right.
3) Solve.
This is a shortcut method, so there is a simple way to solve this proportion, that does the same math you would do when using the traditional conversion factors or other methods, but faster.
First, find where your x variable is (the quantity that you want to find). Then, multiply the 2 numbers that form a diagonal line above the x. (Usually, it is number that is above x, and the number next to x). Then, divide by the number diagonally across from x.
In this case, you would multiply 1 (the number above x) by 37.15 (the number next to x) and divide by 44.01 (the number across from x). I like to draw a circle around the first two digits so they form a diagonal line, so it is visually easier to comprehend what to multiply and divide.
The equation would look like this:
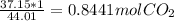
So now we know that 37.15 grams of CO2 is equal to 0.8441 moles of CO2.
4) Set up the proportion to find how many moles of oxygen are required to produce 37.15 g of CO2.
Looking back at the original balanced equation, C3H8 + 5O2 -> 3CO2 + 4H20, we can use the number of moles to set up a reactant:product ratio.
O2: CO2
5 mol: 3 mol
With this information, we can set up a proportion, using the same method from before:
5 mol O2 - 3 mol CO2
x mol O2 - 0.8441 mol CO2
This proportion shows that the ratio of 5 mol: 3 mol should be the same as x mol: 0.8441 (the number of moles in 37.15g CO2) mol.
5) Solve.
Multiply the 2 numbers that form a diagonal line above x. (the number above x and the number next to x)
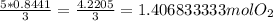
Finally, round the digit to 1.407 mol.
1.407 moles of oxygen are required to produce 37.15 g of carbon dioxide.