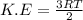Chemistry, 27.09.2019 03:30 maxwellirowa10oy9yaf
What is the relationship between the kinetic energy of molecules in an object and the object's temperature?
as the temperature increases, the kinetic energy of the molecules increases
the kinetic energy always increases whether the temperature increases or decreases
the total kinetic energy of the molecules is not affected by a change in temperature
as the kinetic energy of the molecules decreases, the temperature increases

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
What is the relationship between the kinetic energy of molecules in an object and the object's tempe...
Questions

History, 14.06.2020 06:57




Mathematics, 14.06.2020 06:57


Mathematics, 14.06.2020 06:57




Health, 14.06.2020 06:57

Mathematics, 14.06.2020 06:57


Mathematics, 14.06.2020 06:57


Mathematics, 14.06.2020 06:57