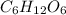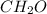Chemistry, 28.10.2019 17:31 joannamarquez0701
If a compound has a molar mass of 180 g/mol and its empirical formula is ch2o, what is its molecular formula?
ch2o
c2h4o2
c6h12o6
c12h22o11

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3

Chemistry, 23.06.2019 11:00
The lab procedure involves several factors, listed below some were variable and some were constant. label each factor below v for variable ot c for constant
Answers: 1

Chemistry, 23.06.2019 15:30
An isotope undergoes radioactive decay. the new isotope that forms has an atomic number fhat is 2 less than the original isotopes. which kind of decay has occured and how do you know
Answers: 2

Chemistry, 23.06.2019 17:30
How do you determine the degree of a power function from a table? when do you know you have successfully determined the degree of the power function?
Answers: 1
You know the right answer?
If a compound has a molar mass of 180 g/mol and its empirical formula is ch2o, what is its molecular...
Questions



Geography, 25.10.2020 22:00

Mathematics, 25.10.2020 22:00

History, 25.10.2020 22:00

Computers and Technology, 25.10.2020 22:00

Mathematics, 25.10.2020 22:00


English, 25.10.2020 22:00


Chemistry, 25.10.2020 22:00




Mathematics, 25.10.2020 22:00

Mathematics, 25.10.2020 22:00


History, 25.10.2020 22:00