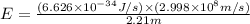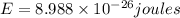Calculate the change in energy of an atom that emits a photon of wavelength 2.21 meters. (planck’s constant is 6.626 x 10-34 joule seconds, the speed of light is 2.998 x 108 m/s)
* 8.9886 x10-26 joules
*4.8844 x 10-42 joules second
* 1.9864 x 10-25 joules
* 1.4643 x 10-33 joules /second

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1


Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1

Chemistry, 23.06.2019 03:30
Ineed pls urgent 1-20 in order and fully detail step my step.
Answers: 1
You know the right answer?
Calculate the change in energy of an atom that emits a photon of wavelength 2.21 meters. (planck’s c...
Questions



English, 25.09.2020 14:01

SAT, 25.09.2020 14:01



History, 25.09.2020 14:01

Advanced Placement (AP), 25.09.2020 14:01

Advanced Placement (AP), 25.09.2020 14:01

English, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01

Mathematics, 25.09.2020 14:01

Advanced Placement (AP), 25.09.2020 14:01


Health, 25.09.2020 14:01


Social Studies, 25.09.2020 14:01


Mathematics, 25.09.2020 14:01

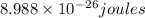
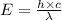
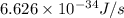
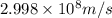
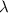 = wavelength = 2.21 m
= wavelength = 2.21 m