
Chemistry, 24.01.2020 08:31 natalie9316
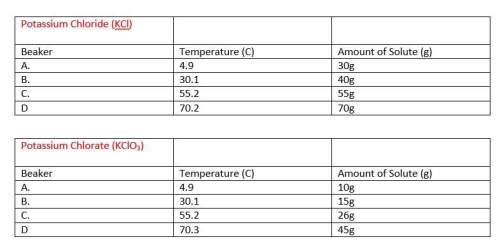
1)describe in a step by step manner how you determined the identity of the two unknowns. discuss both what you determined the unknowns to be and the method that you used to find them.
2)use your knowledge of collision theory to explain the results of your experiments in this laboratory.




Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
You know the right answer?
1)describe in a step by step manner how you determined the identity of the two unknowns. discuss bot...
Questions

Mathematics, 02.12.2020 01:40

Mathematics, 02.12.2020 01:40

Mathematics, 02.12.2020 01:40

Mathematics, 02.12.2020 01:40


Computers and Technology, 02.12.2020 01:40

Physics, 02.12.2020 01:40





Chemistry, 02.12.2020 01:40


Mathematics, 02.12.2020 01:40


Mathematics, 02.12.2020 01:40



English, 02.12.2020 01:40

Mathematics, 02.12.2020 01:40



