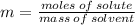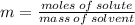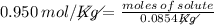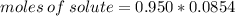Chemistry, 10.10.2019 12:30 raymondanthony6567
The molality of a solution that is made by dissolving a certain mass of ethanol in 85.4 g of water is 0.950 m. how many moles of ethanol were dissolved?
0.0811 mol
0.0899 mol
11.1 mol
81.1 mol

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

You know the right answer?
The molality of a solution that is made by dissolving a certain mass of ethanol in 85.4 g of water i...
Questions

Mathematics, 20.01.2021 01:00

Mathematics, 20.01.2021 01:00


Biology, 20.01.2021 01:00

Mathematics, 20.01.2021 01:00




Mathematics, 20.01.2021 01:00

Mathematics, 20.01.2021 01:00




Mathematics, 20.01.2021 01:00

History, 20.01.2021 01:00



Mathematics, 20.01.2021 01:00


History, 20.01.2021 01:00