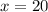Chemistry, 04.02.2020 15:50 applereams
Boron has an atomic mass of 10.8 amu. it is known that naturally occurring boron is composed of two isotopes, boron-10 and boron-11. determine the percent of each isotope of boron


Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
Boron has an atomic mass of 10.8 amu. it is known that naturally occurring boron is composed of two...
Questions


Mathematics, 21.05.2020 02:59

History, 21.05.2020 02:59

Mathematics, 21.05.2020 02:59





History, 21.05.2020 02:59



Mathematics, 21.05.2020 02:59





Mathematics, 21.05.2020 03:00



Mathematics, 21.05.2020 03:00

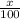
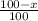
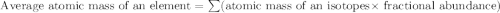
![10.8=\sum[(10)\times \frac{x}{100})+(11)\times \frac{100-x}{100}]]](/tpl/images/0500/8694/6044f.png)